Master Validation Plan Template - Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web $ 75.00 add to cart the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web three (3) options to create a validation master plan.
Validation Master Plan Template Verification And Validation Pharmaceutical
Web $ 75.00 add to cart the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web three (3) options to create a validation master plan. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web a free master validation plan (mvp).
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list.
Overview of the Validation Master Plan PresentationEZE
Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web $ 75.00 add to cart the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web a validation.
Validation Master Plan Template Validation Center
Web $ 75.00 add to cart the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and. Web three (3) options to create a validation master plan. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web a validation master plan (vmp) is a document.
Validation Master Plan (VMP) Downloadable Interactive Template.
Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web three (3) options to create.
What is Validation Master Plan? (Template, Examples)
Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web.
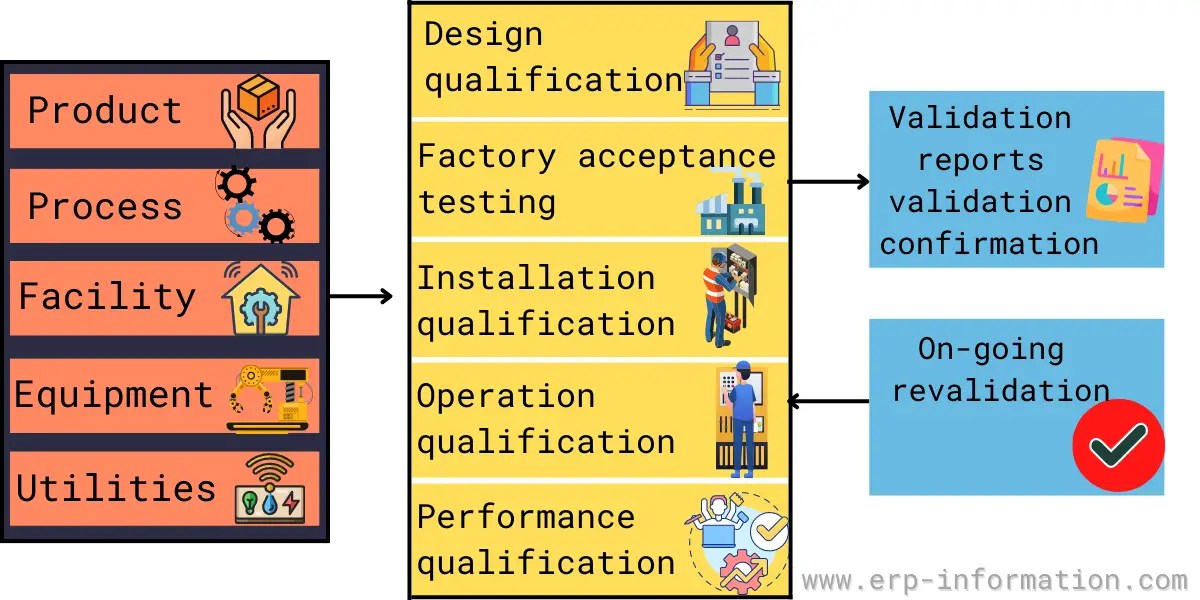
Nine steps for creating a Master Validation Plan
Web $ 75.00 add to cart the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes. Web three (3) options to create a validation master plan. Web a master validation plan.
Web a master validation plan (mvp) is a plan for your equipment and process validation activities. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes. Web $ 75.00 add to cart the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web three (3) options to create a validation master plan.
Web Three (3) Options To Create A Validation Master Plan.
Web $ 75.00 add to cart the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and. Web creating a master validation plan template for medical devices with procurement introduction to master validation plan. Web a validation master plan (vmp) is a document that details the way a company will operate, who has control over the various aspects of the. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes.